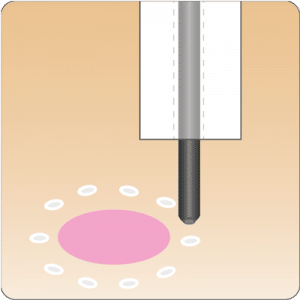
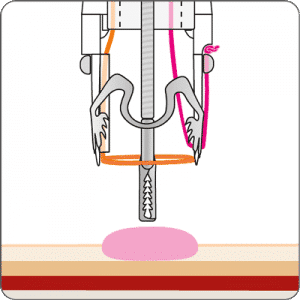
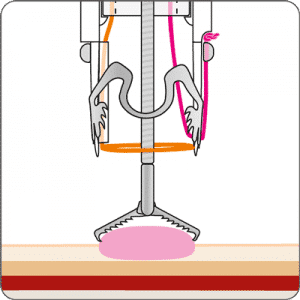
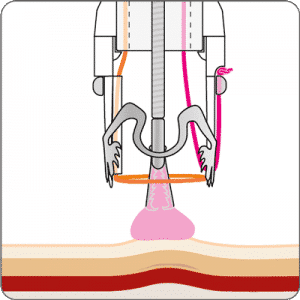
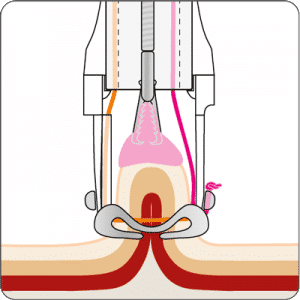
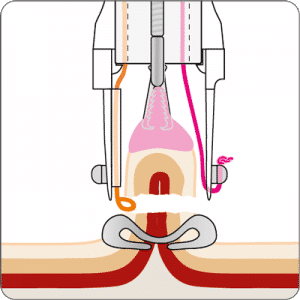
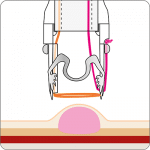
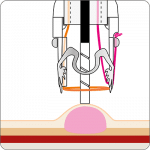
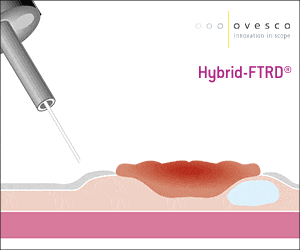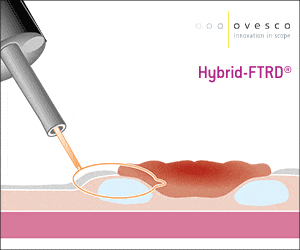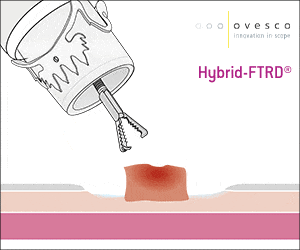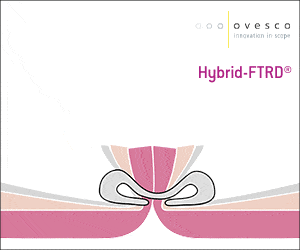
The gastroduodenal FTRD® enables endoscopic full-thickness resection in the stomach and duodenum.
+49 (0) 7071 96528 160
service@ovesco.com

+49 (0) 7071 96528 160
service@ovesco.com
A smaller FTRD® System for endoscopic full-thickness or deep wall resection (especially in the stomach) and diagnostic tissue acquisition in the stomach and duodenum.
The gastroduodenal FTRD® can be used for:
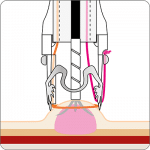
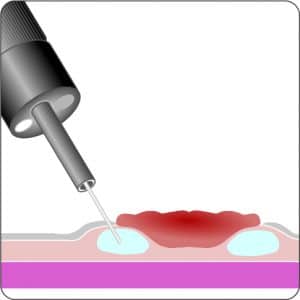
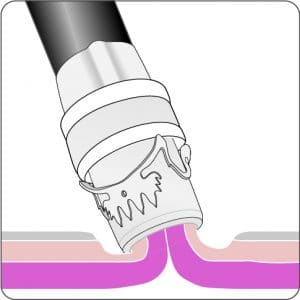
The gastroduodenal FTRD® consists of an application cap with a ready-to-use mounted FTRD® clip, integrated HF snare and thread, thread retriever, the endoscope sleeve with fixation tapes and FTRD® hand wheel.
For an easier and safer introduction of the system into the upper GI-tract (passage of esophagus and pylorus) the gastroduodenal FTRD® Set is delivered with an insertion balloon and guide wire.
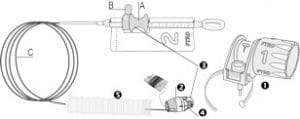
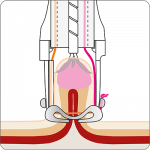
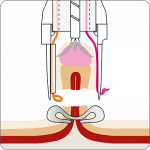
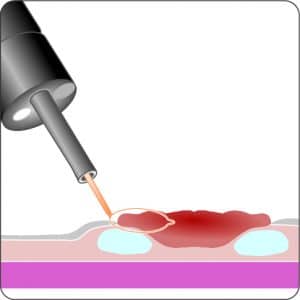
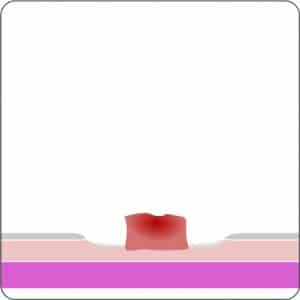
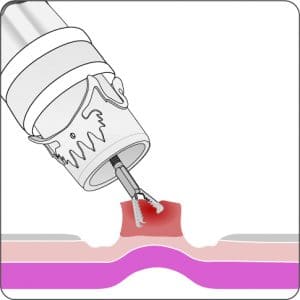
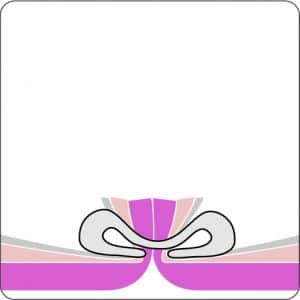
For application, the application cap is mounted on the endoscope with the snare running on the outside of the scope and the sleeve preventing entrapment of any tissue between scope and snare. By turning the hand wheel, the thread is tensioned and the clip released. Using the integrated snare, the target tissue is cut above the clip.
The size of the gastroduodenal FTRD® is suitable for endoscopes with a diameter of 10.5 – 12.0 mm and a working channel diameter of at least 3.7 mm.
The gastroduodenal FTRD® is delivered as one procedure set and consists of the following products:
For a better mobilization of the tissue in case of submucosal findings (especially in the stomach) an Anchor is available as a separate item (not included in the procedure set).

Before purchasing and using the FTRD®, participation in a training course is mandatory.
| Cap diameter (outside) [b] | 19.5 mm |
| Cap diameter (inside) [c] | 12.1 mm |
| Cap depth | 23 mm |
| Appropriate endoscopes | Endoscop diameter: 10.5 – 12.0 mm Working channel diameter: min. 3.7 mm |
| Packaging unit | 1 piece included in gastroduodenal FTRD® Set |
| Reference number of gastroduodenal FTRD® Set (incl. FTRD® Grasper, FTRD® Marking Probe, guide wire and insertion balloon) | 200.72 |
The gastroduodenal FTRD® can be used with endoscopes with an outer diameter between 10.5 – 12.0 mm and a working channel diameter of at least 3.7 mm. All FTRD® products are disposable and designed for single patient use.